Note: This post is the third in a four-part series on Ireland’s COVID response (read parts one and two first). Any fact that isn’t linked is sourced from the book Pandemonium: Power, Politics and Ireland’s Pandemic.
3.1. We should have used human challenge trials
The greatest triumph of the pandemic is, of course, that the vaccines are highly effective and were developed more than five times quicker than the next quickest vaccine. Huzzah! The only reason I’m not making a bigger deal out of this is that Ireland can’t exactly take credit for it. But it can take the blame for failing to use human challenge trials, i.e. testing whether the vaccine works by deliberately infecting people with Covid-19. HCTs assess a vaccine’s efficacy much quicker than is possible with a Phase III clinical trial, which involves simply waiting around to see how many people get Covid. HCTs would have sped up the vaccines’ development by months. It’s impossible to know how many lives they would have saved; perhaps millions. Their most important role may have been to determine the minimum doses necessary and thus massively expand supply. To my knowledge, no one prominent in Ireland even brought up HCTs, but, judging by the experience of other countries, they would have been dismissed for ethical reasons and a general unwillingness to consider unorthodox ideas. If the problem is that volunteers aren’t compensated for the risks they take on, then simply pay them. Surveys find that the public is strongly in favour of HCTs, and even Christine Korsgaard, one of the leading scholars on deontological ethics, signed a petition in favour of their use. Common sense prevails over complicated and unintuitive moral theories: it’s good to allow people to participate in studies which will save many lives.
The good news is that, thanks to heroes with infinite patience, human challenge trials are more likely to be used for future pandemics. And the UK did eventually run an HCT, though it was too late to be very useful. Notably, that trial was run by an Irish company.
3.2. The vaccines were approved too late
The vaccines were approved too late. This is a totally separate point to the use of HCTs: even conditional upon deciding to run long Phase III trials, the vaccines should have been approved based on preliminary data, rather than waiting for weeks after the announcement of results. Regulatory agencies were at this time a cesspool of moral error. The FDA “and its approximately 17,000 employees were dark for the four-day Thanksgiving holiday, including those working on the vaccine approval.” Various medical experts are convinced that the data analysis was sufficiently straightforward that it shouldn’t have taken more than 24 hours. I’m not claiming that the authorities literally weren’t working hard. I’m sure they worked long hours. It’s just that the work done in those hours was, in the context, actually enormously harmful. Europe was only a smidgen better.
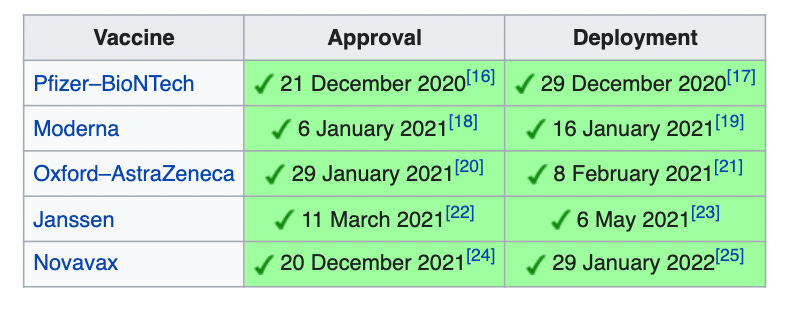
European drug regulation is mostly handled at the EU level, through the European Medicines Agency, which is very similar to the FDA in America. There are two ways a drug can be approved in Europe. First, through a centralised procedure where, if the European Commission approves the drug, it becomes legal in the EU and the European Economic Area. This is mandatory for certain innovative treatments, especially ones involving rare diseases. Second, the EMA can approve a drug, with national authorities having to re-approve it, which they often don’t do, e.g. the antidepressant tianeptine is available in France but not Ireland. My understanding is national regulatory bodies can in principle approve drugs that aren’t approved by the EMA, but they are heavily dissuaded from doing so. The Covid vaccines were approved through the first route: Ireland was legally bound to allow them. But in practice, the government waited for an advisory sign-off from the National Immunisation Advisory Committee. This is why Ireland was able to pause AstraZeneca even though the EMA knew it was a bad idea. Here’s the table from Wikipedia of the vaccine timeline in Ireland. The Pfizer, Moderna and AstraZeneca sign-offs were sufficiently fast to not be consequential. But NIAC delayed for weeks before signing off on the Janssen (Johnson & Johnson) vaccine.
Boosters were also approved far too late. It would have been reasonable to delay boosters to distribute supply to the developing world, but this is not what happened. In per capita terms, Ireland donated fewer vaccines than the EU average, itself a fairly pitiful amount. Boosters took so long because of the intense risk-aversion we’ve by now become familiar with. And variant-specific boosters still haven’t been approved. Boosters against Delta or Omicron would have averted many cases and deaths. The bottleneck preventing this was not the companies themselves but regulators dragging their feet. Could Ireland, or another European country, have unilaterally decided to approve a vaccine much sooner than its peers?
It may technically have been possible, but it would have been very difficult. It’s exactly what Hungary did. In March 2021, it approved the Sinopharm, Sputnik V and CanSino vaccines, leading to an initially faster rollout than most of Europe. I think Ireland could have done something similar, but in practice, it would have been politically impossible. It is probably not a coincidence that these vaccines were approved in a country that is so alienated from the rest of the EU.
3.3. We underpaid for vaccines
Ireland was quick to introduce a welfare scheme for those that had been laid off because of Covid. The Pandemic Unemployment Payment was €350 per week, which was substantially higher than many part-timers were making before they were unemployed. The scheme was still open until January this year. Ireland’s spending was unbelievably loose, and yet penny-pinching elements objected to free Covid tests and “overpaying” for vaccines.
Holohan considered free antigen tests in the UK a waste of money, even as both countries were pumping billions into pandemic social programmes of questionable value. The EU as a whole displayed great concern that it did not overpay for vaccines. It waited three months after the UK to sign a deal with AstraZeneca to haggle down prices (AstraZeneca reported that they literally didn’t make any profit by selling to the EU). They delayed Pfizer negotiations by weeks for the same reason, and concerns over liability.
The most pernicious effect of this miserliness was to dry up supply for the developing world. Manufacturing and distributing vaccines is extremely capital intensive. While it is completely natural to be concerned about pharmaceutical companies making too much money from the pandemic, an economist would be more concerned about them not making enough money. The simple reason is that, when there are positive effects on other people, the market value of a good will be less than its social value. The EU paid only €12 for a dose of Pfizer. But the social value of the vaccines was absolutely enormous: certainly in the thousands per dose. If anything, we should be paying people to get vaccinated!
The creation of medical products is typically incentivised with patents. This creates serious equity concerns, as the company can overcharge for and undersupply its product. Solutions to this include patent buyouts and prizes, in which the government pays companies to design a product of a certain specification. In any case, the vaccine companies gave their licenses away for free and ran out of manufacturers to give them to. Likely patents weren’t the problem in this specific instance. When patents aren’t doing a good enough job incentivising production, or they are likely to be waived, a solution is to offer advance market commitments: offering in advance to buy the vaccines at a certain price. These were used to great effect in the pandemic.
None of the Covid vaccines were available to purchase by anyone other than governments, in a purported act of compassion. One of the reasons people in the Global South did not have adequate access to vaccines is that we prevented them from buying any, except through sluggish government purchases and COVAX. I’m not advocating anything close to a free market in vaccines; governments spent orders of magnitude too little on vaccines. But, when you find yourself in a hole, the first thing you do is stop digging. The West displayed tremendous arrogance. We were so sure our system of distributing vaccines was right that we threatened to send people to jail for doing it any other way.
Pandemics are a challenge for finance and the efficient allocation of capital. Ventilators were a key concern in the first wave, but many governments were reluctant to buy them in case they weren’t needed. I would have been willing to pay for a ventilator to keep myself alive, and many other people were also. Yet, for example, the US government relied on a single small contractor for its ventilators, which later pulled out because it wasn’t making them enough money. This is, in theory, a problem of a missing market: a well-designed financial instrument could have matched people’s willingness to pay with the manufacturers – for example, a “ventilator insurance” that would ensure you had access to a ventilator if you needed it. The same goes for ICU beds. When people want something more than it costs to make it, and no one is making more of it, something has gone wrong. This is likely to create waste. But waste is good, if it means you were taking risky bets that had a high payoff. If you’re not missing any flights, you’re spending too long in airports. And if none of your vaccine factories go to waste, you’re not building enough vaccine factories.
3.4. Vaccines were unnecessarily regulated even after approval
I don’t know a single Irish person who isn’t vaccinated, but I do know lots of people who expressed great pickiness over which vaccine they got. This in part arose from governments’ incredibly misleading guidance and messaging.
In January 2021, Emmanuel Macron inexplicably described AstraZeneca as “quasi-ineffective” for older people. Several European countries banned its use in the elderly, before flipping their advice to only allow for its use in the elderly. In February, Ireland banned the use of AstraZeneca in people over 65. Then, in April, AstraZeneca was restricted to only people over 60, before this was undone in June. Ireland handled the J&J shot in a similar way: Johnson & Johnson was for some time forbidden for use in the under-50s.
I’m sceptical of pandemic stories involving social trust. Maybe approving the vaccines sooner would have led more people to distrust them. But maybe the opposite is true. I think social science is in too nascent a state to answer this question. But I strongly suspect that fiddling around with AstraZeneca undermined social trust. Banning use in a group, then un-banning, then banning for the opposite group makes you look incompetent. The UK managed this much better, providing information about rare side effects in context, without restricting who can get what vaccine.
The same misunderstandings of uncertainty were on display during the large delays in approving the vaccines for children. The cost-benefit of vaccination is admittedly rather different for children, but how could it be wise in a pandemic to forbid anyone from getting vaccinated? Even now, parents don’t have the option to vaccinate their children under five. We saw the same thing again when pregnant women weren't allowed to get vaccinated. Sometimes I think that public health experts live in a fantasy land in which everything needs government approval and people aren’t allowed to make decisions that carry risk. These people know that pregnant women can go out and get blackout drunk, right? And that you’re allowed to jump out of a plane just for fun? Apparently, ethical problems only arise when you’re taking a risk to protect yourself from a deadly disease.
The same thing happened again with the approval of ‘mix-and-match’ vaccines. Boosters in Ireland are only allowed to be Pfizer or Moderna, and you weren’t allowed to be vaccinated with two different types of vaccines in your initial course. It looks like mixing vaccine types is more protective, but it’s too complicated for me to weigh in on. What I object to is the schizophrenic state of affairs in which we go from some public health measure being forbidden to obligatory, as if we were following The Science all along. And there is never any admission of error. We have always been at war with Eastasia.
The vaccine campaign was very quick once it got underway. I would be surprised if many people died as a result of the pauses or the delayed approval for children and pregnant women. But it certainly caused a large number of unnecessary cases. And all were illustrative examples of the government doing a poor job handling risk and uncertainty.
3.5. The interval between doses was too short
The United Kingdom’s vaccine rollout initially enjoyed great success in part because they expanded supply by spacing out the intervals between the two doses of Pfizer, Moderna, and AstraZeneca to twelve weeks. While Ireland had a twelve-week interval for AstraZeneca, it stuck to the four weeks that the initial Phase III trials were conducted over for Pfizer and Moderna (note that the vast majority of vaccines administered in Ireland were Pfizer). There was also initially a policy of holding second doses in reserve, which slowed down the rollout. If you had two children and two vaccines, would you give them both 70% immunity, or give only one 95% immunity? Would you hold the second dose in reserve for a month while one child went totally unprotected? The UK’s strategy came to be known as ‘First Doses First’ and was subsequently endorsed by a wide range of experts and adopted in several other countries. First Doses First expands the supply of vaccines in the short term, but throughout the Spring and Summer, it became clear that longer intervals actually generated a better immune response. As it turns out, Delta substantially lessened the value of First Doses First but this poor decision-making was another example of uncertainty turning our collective brains to mush.
The UK was vaccinating much quicker than the rest of Europe, and this led to a diplomatic crisis in which the EU evoked Article 16 of the Northern Ireland protocol to limit exports of vaccines to the UK. This was reversed within hours but did sour relations significantly. ‘Bullying’ is an uncharitable word to describe this, but it is not inaccurate.
3.6. Too few drugs were tested
In principle, the four main ways to fight a pandemic are vaccines, lockdowns, tests, and drugs. Irish people could apparently only keep two of these in our minds at a time, or three at a stretch. The most commonly neglected way was drugs, or other tools which make severe outcomes less likely conditional upon getting sick. The body works in mysterious ways, and it’s common for drugs designed for one purpose to end up serving a completely different one. Famously, Viagra started off as a blood pressure medication. We should have thrown the kitchen sink at Covid to test what works. What we got instead was too few trials too late with small sample sizes.
Once again, the UK did better than Ireland on this front. The RECOVERY trial run by the University of Oxford had 40,000 participants. This is what established that hydroxychloroquine almost certainly doesn’t work. It also found that dexamethasone, a cheap steroid, reduces Covid deaths by a third. It identified tocilizumab and baricitinib as drugs that reduce the death rate, albeit by less than dexamethasone. Another promising drug is the antidepressant fluvoxamine, which was identified in the TOGETHER trial (for some reason, every Covid clinical trial has a cutesy acronym). Canada has approved it but most other countries haven’t. Sadly, Ireland didn’t run anything near the scale of the RECOVERY trial.
A previous essay on the Fitzwilliam argued that Ireland should fill a niche by becoming the first county with sensible nuclear regulation. Perhaps an even more valuable niche to fill would be to make Ireland the global hub of clinical trials. There are many ways that we can expedite Phase I and II trials, ethics review and recruitment. This would be selfishly worth it for Ireland, giving us quick access to the best vaccines and treatments in emergencies. But the benefits for the rest of humanity would be enormous.
The small sample sizes of Covid trials are sometimes baffling. The aforementioned human challenge trial in the UK only had ninety participants. This is despite the fact that they had 40,000 volunteers sign up. What could the authors possibly have been working on that was more important than recruiting more participants to the study?
Large sample sizes are important for two reasons. First is that, when you’re thinking about rolling out a drug on a wide scale, even small reductions in uncertainty are worth a lot. Second is that larger samples allow you to detect subgroup effects. Ninety people are not enough to reliably determine if there’s a different effect by age, gender, race, or pre-existing condition. Although it feels like an eternity ago, Covid’s disparate racial outcomes were once a common topic of discussion. These questions could have been relatively easily answered with large enough studies.
If I may put on my speculating hat, I think the reason why there were so many trials with small sample sizes is that academics are incentivised to generate as many papers and novel findings as possible. Ninety people may be enough to demonstrate an interesting biological phenomenon and win you prestigious research grants. But it’s not enough to test drugs or vaccines in an emergency situation. Now, scientists aren’t evil; they did not consciously decide it was more important to advance their careers than to generate useful information about Covid treatments. But the truth is that there are probably few academics that have the money, time, and logistics expertise to run a trial with 40,000 participants.
I hope that readers are inspired rather than depressed by reading about these failures. Academics aren’t just making mistakes. They are making mistakes so glaring that a teenager in her bedroom can figure them out. This is not remotely to imply that the solutions are simple. Just that the space of possible improvement is truly vast.
Sam Enright is executive editor of the Fitzwilliam.


Hi Sam, this is a very complicated topic. Ive been working in technology transfer for over a decade and in biopharma for years before that. In general I agree with a lot of what you're saying, especially regarding patent waivers. Although big pharma does hide behind the complexity of manufacturing sometimes also. Anyway, like many, you've completely skipped the role of universities/institutes in IP. I wrote this a couple of years ago. May be of interest.
https://www.irishtimes.com/business/health-pharma/university-know-how-the-secret-sauce-for-vaccine-success-1.4477006
Interesting article but I think you're a bit gung ho with the vaccine roll out. They have to be constantly monitored for safety so going too quick, even in a pandemic, could end up creating more problems than it solves.